Questions About Biocompatibility for Medical Devices Answered
- the general principles governing the biological evaluation of medical devices within a risk management process;
- the general categorization of medical devices based on the nature and duration of their contact with the body;
- the evaluation of existing relevant data from all sources;
- the identification of gaps in the available data set on the basis of a risk analysis;
- the identification of additional data sets necessary to analyze the biological safety of the medical device;
- the assessment of the biological safety of the medical device.
- the patient's body during intended use;
- the user's body, if the medical device is intended for protection (e.g., surgical gloves, masks and others).
- risks, such as changes to the medical device over time, as a part of the overall biological safety assessment;
- breakage of a medical device or medical device component which exposes body tissue to new or novel materials.
- Physico-chemical tests
- Fourier-transformed infrared spectroscopy (FT-IR)
- Analysis of organic substances
- Analysis of inorganic substances
- Physical mechanical analysis
- Molecular weight distribution
- Morphological characterization
- Thermal Analysis
- ASTM F756: Standard Practice for Assessment of Hemolytic Properties of Materials
- ASTM F720: Standard Practice for Testing Guinea Pigs for Contact Allergens: Guinea Pig Maximization Test
- ISO 10993-4: Biological evaluation of medical devices - Part 4: Selection of tests for interactions with blood
- ISO 10993-5: Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity
- ISO 10993-10: Biological evaluation of medical devices - Part 10: Tests for irritation and skin sensitization
- ISO 10993-11: Biological evaluation of medical devices - Part 11: Tests for systemic toxicity
- USP : Pyrogen Test
- ISO 10993-12: Biological evaluation of medical devices - Part 12: Sample preparation and reference materials
All medical devices that have direct or indirect contact with the human body require an assessment of the Biocompatibility of the materials used to manufacture the device, with the test schedule depending on the nature and duration of body contact.
Medical device executives need to understand all the regulatory compliance requirements for bringing a device into the market including a thorough understanding of the material characteristics and biocompatibility. Domestic and international regulatory bodies emphasize the use of risk-based approaches to assess Biocompatibility.

What is Biocompatibility?
The Merriam Webster Dictionary defines Biocompatibility as "compatibility with living tissue or a living system by not being toxic, injurious, or physiologically reactive and not causing immunological rejection."
What is Biocompatibility testing?
Biocompatibility testing refers to testing to determine the "potential for an unacceptable adverse biological response resulting from contact of the component materials of the device with the body." It measures how compatible the finished device is with a biological system.
Why is Biocompatibility testing critical?
Biocompatibility is a key aspect of the overall safety profile of medical devices.
To ensure patient and staff safety
A medical device might cause local or systemic effects directly or indirectly or through the release of its chemical components. For example, it could cause local effects such as skin irritation, pain, or burns, or systemic effects such as developmental effects, reproductive system effects, or cancer. To prevent such harm to users, biocompatibility testing is critical.
To ensure proper functioning of the device
The physical and chemical characteristics of materials used in the manufacture of the medical device can cause an indirect impact on the functionality of the device. For instance, chemicals that leach from the device can change the electronic or mechanical properties of the device. Also, processes such as manufacturing, packaging, sterilization, and aging can impact the material's composition.
To comply with regulatory requirements and gain regulatory approvals
The U.S. Food and Drug Administration (FDA) requires device manufacturers looking to obtain pre-market approval under its 510(k) program to submit testing data authenticating the biocompatibility of any device or material that comes in direct or indirect contact with a patient. Failure to provide the data or inadequate data submission may result in rejection of a 510(K) submission, and delay the approvals.
To save costs
Proper biocompatibility testing increases the speed of product development, market introduction, and prevents the potential for lost revenues.
What are the regulatory standards for the biocompatibility of medical devices?
ISO 10993 Suite
Contain standards that cover all testing procedures under "Biological evaluation of medical devices"
ISO 10993 -1
This document specifies:
This document applies to evaluation of materials and medical devices that are expected to have direct or indirect contact with:
This document is applicable to biological evaluation of all types of medical devices including active, non-active, implantable and non-implantable medical devices.
This document also gives guidelines for the assessment of biological hazards arising from:
Other parts of ISO 10993 cover specific aspects of biological assessments and related tests. Device-specific or product standards address mechanical testing.
This document excludes hazards related to bacteria, moulds, yeasts, viruses, transmissible spongiform encephalopathy (TSE) agents and other pathogens."
US FDA Guidance Document
Use of International Standard ISO 10993-1, "Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process," also referred to as the FDA's Biocompatibility Guidance on Use of ISO 10993-1
MDR
Regulation (EU)2017/45 of the European Parliament and of the Council of 5 April 2017 on medical devices
What specific biocompatibility tests should be conducted?
The tests that should be conducted depend on the category of the medical device, its intended use, and duration of contact between the device and the body.
What are the various test procedures that are used as part of the material characterization process?
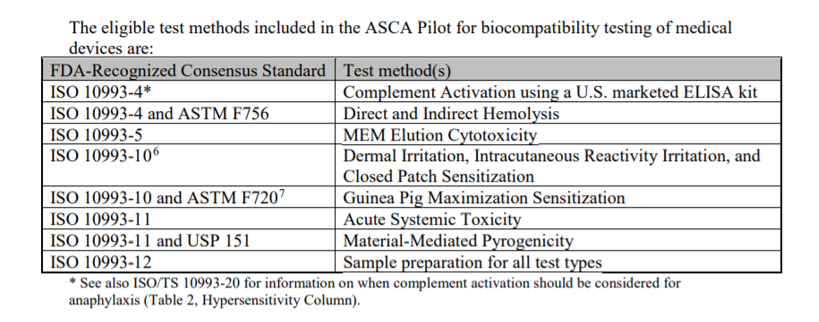
Enlist the FDA-Recognized Consensus Standards and Test Methods in the ASCA Pilot for Biocompatibility Testing of Medical Devices
What are the eligible test methods included in the ASCA Pilot for biocompatibility testing of medical devices?
Source: Biocompatibility Testing of Medical Devices - Standards Specific, Information for the Accreditation Scheme for Conformity Assessment, (ASCA) Pilot Program
Looking for related training? Check out our Seminars and Webinars for FDA-regulated Companies.