Understanding Biotechnology IT Regulatory Compliance
IT compliance in Biotechnology is centered on the regulatory requirements of the industry's regulatory bodies. It is practiced to satisfy regulatory requirements while facilitating business operations. IT Compliance is driven by business needs rather than technical needs.
Biotech companies must play by the rules. Failure to comply with the regulations can result in exorbitant fines and penalties. It can put the company, the jobs of its employees, and the dividends of its shareholders at stake. Competitors can take an upper-hand by taking advantage of the compliance failure problems of the company.

The 3 Pillars of an ongoing and flexible risk management strategy for IT compliance
Regulated Biotech companies manage a massive amount of data. They are required to handle and store that data in compliance with the guidelines, specifications, and regulations set forth by the Food and Drug Administration (FDA) and the U.S. Department of Health and Human Services (HHS). Everything from the WIFI to computer-based systems must be in harmony with the guidelines.
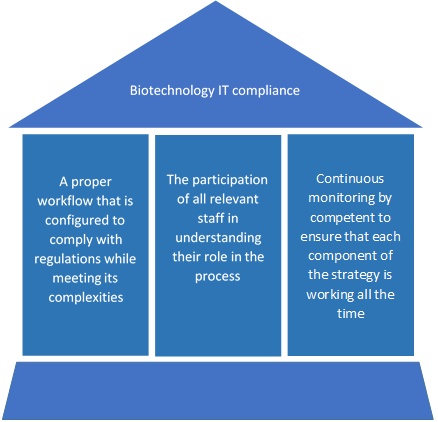
Given that technology is constantly evolving, IT compliance is a constant challenge for Biotech companies. It requires a sustainable risk management strategy that requires the following 3 pillars:
- A proper workflow that is configured to handle the complexities of IT compliance
- The participation of all relevant staff in understanding their role in the process
- Continuous monitoring by qualified and competent IT professionals who understand the workflow to ensure that each component of the strategy is working as they should all the time.
These pillars will provide confidence that a company can successfully pass the compliance audit.
- The Regulatory Bodies
- Agriculture and Farming
- Veterinary Medicine
- Any Branch of Biomedicine and Biotechnology
- Drug and Supplement Manufacturing
- Cosmetics
- Medical Research
- Development and Manufacture of Vaccinations, Blood, and Tissue Products
- Any Branch of Biomedicine and Biotechnology
- Medical Equipment and Devices
- Equipment or Devices that Emit Radiation
- The Security Rule
- The Unique Identifiers Rule
- The Enforcement Rule
- The Privacy Rule
- The Transactions and Code Sets Rule
- The understanding of the reasons for the HIPAA act and how it relates to individual responsibilities
- A proactive approach to compliance strategy, implementation and monitoring to ensure that personal health data is transmitted, stored, and used the way it is required to be.
- A Partial list of useful resources for IT compliance
- Current Concepts and Challenges in Cloud Compliance
- Validation and Use of Cloud Computing in FDA Regulated Environments
- Implementation and Management of GMP Data Integrity
- Computer System Validation and Part 11 Compliance
- Risk-Based Approach in cGMP - Quality Risk Management (QRM)
- Critical Elements of Quality Risk Management Relating to FDA Compliance
- Technical Writing for Medical Products: SOPs, Investigations and Change Records
- Controlled Document System for a Life Sciences Manufacturing Plant - Principles of Lean Documents and Lean Configuration
- Good Documentation Guideline (Chapter <1029> USP)
- Technical Writing for the Pharmaceutical, Medical Device and Biotech Industries
- How to prepare a winning Standard Operating Procedure (SOP)
- Some advantages of Biotechnology IT compliance
- Bolster an organization's reputation resulting in winning new business with customers who are security-minded.
- Help in finding the gaps in Internet Security (IS) program which otherwise might not have been identified outside of a compliance audit
- Help have a standardized security program, as opposed to being only configured by the administrator's choice.

The Food and Drug Administration and the U.S. Department of Health and Human Services are the 2 big regulatory agencies involved in the compliance for biotechnology companies. Depending on the work whether the work involves food, pharmacological research, or medical research, the company may fall under one or both of these agencies.
The FDA
The FDA supervises the country's food chain and the development, testing, and approval of drugs. With a goal to safeguarding the public, the agency acts as a control board.
Companies that are involved in the following fall under FDA's jurisdiction
The documentation required of your company is defined in a manual of procedures. The manual published by a sub-section of FDA known as the Center for Drug Evaluation and Procedures (CDER). Your company must document your internal policies and procedures in alignment with the Federal Food, Drug, and Cosmetic Act other relevant laws enforced by the FDA. This documentation should be submitted to the FDA as proof of your compliance with the policies set forth by the agency.
The standards FDA has set forth for Biotech companies are considered by the Government as the bare minimum that your company should abide to protect the public. It is prudent for your IT compliance staff to receive training in Biotechnology Documentation and IT Regulatory Compliance to ensure that your systems are in compliance and exceed expectations.
HHS
The Health Insurance Portability and Accountability Act (HIPAA) is a collection of sets of individual laws. Companies that collect, store or use the private health information of individuals are required to use, store, and secure such data in compliance with HIPAA.
The HIPAA protocols for transmission of electronic healthcare transactions is found in Title II of the laws. Violations of HIPAA statutes include severe penalties which possibly include civil and/or criminal prosecution.
Businesses that fall under HHS and HIPAA are those that have access to private health information of individuals. These include hospitals, clinics, insurance agencies, and companies that do business with healthcare companies and have access to such sensitive customer data. Those companies which fall under HIPAA must have knowledge about the five rules within the HIPAA.
The requisites for a Biotech company to become HIPAA compliant
21 CFR 211.22 requires quality control units to document their policies and procedures in writing. Controlling procedure documents on the manufacture, processing, package, and holding of biotechnology products with a manual process can be extremely cumbersome. The following resources provide insights into documentation and control.
Biotechnology compliance efforts have a useful purpose and are an asset to the business.
Compliance can:
Hundreds of companies use ComplianceOnline platform to educate their staff on biotechnology documentation and IT compliance to prevent and detect violations by employees that could lead to legal liability for the individual and the organization. Join them!






