Delay and Detention of FDA Import Products - Notices, Documents, and FDA interactions solution
Knowledge is power. By understanding the underlying reasons for delay and detention of FDA import products, you can be well-prepared to cut-short or overcome such.

Electronic Screening

Before FDA regulated products enter the U.S, they are electronically screened to ensure that they meet the U.S standards. PREDICT is the software program used in the screening process. If the electronic information you entered meets the required standards, the screening program issues a Notice of action (NOA) that the shipment may proceed.
The software though randomly selects shipments to verify if the information entered is accurate. If errors are found, the information will be reviewed manually for each line item.
OASIS is FDA's software program that targets products with a higher probability of not meeting FDA requirements. This program can process information quickly and issue the NOA.
During the screening, if errors are found in entries, it may cause delay or detention in shipment. Make sure your entries are compliant and correct to avoid such a delay.
Be prepared
If there is a problem, prompt action is required. The firm should be well- prepared and have established protocols for any type of NOA. Being prepared cuts further delays and potential detentions.
To understand the procedures for various types of NOAs, refer the FDA's Regulatory Procedure Manual (RPM.). RPM helps you know what FDA is planning to do. The RPM includes procedures for FDA personnel to follow when FDA is notified of an entry and explains procedures to follow for each type of NOA.
May Proceed Notice

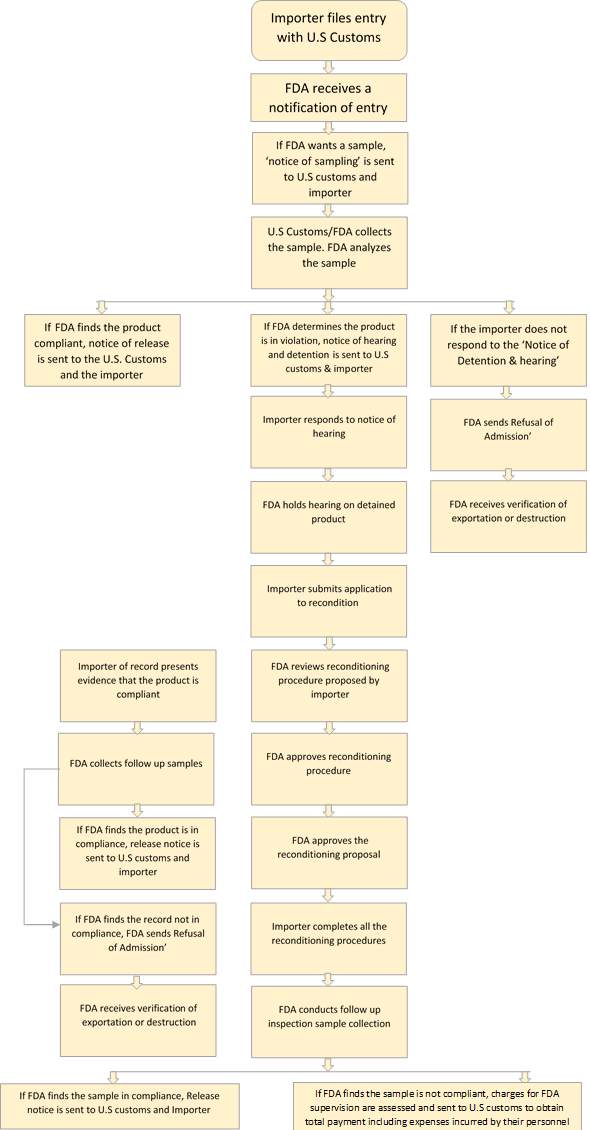
Notice of Sampling: The FDA may collect a physical sample and conduct a field exam where the product is located.
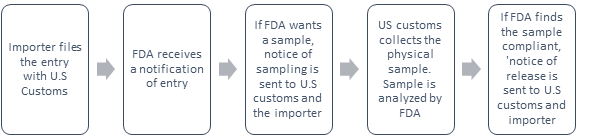
Notice of detention and hearing: If FDA sampling indicates the product appears to be in violation, a notice of detention is sent to the U.S. customs and the importer.
If FDA decides to detain the product, the Notice will specify the nature of the violation. The FDA may subject the product owner or consignee for an informal hearing to present evidence on why the product should be allowed entry or why it can be reconditioned. Unless there is a compelling reason, the FDA will not give the consignee more than 10 working days to provide FDA with testimony or evidence to rebut FDA's position. Usually, the 10-day rule is strictly followed.
The importer can either decide to make the product compliant with FDA requirements or be removed from the FDA's authority. Avoid delay by taking prompt action if you decide to make the product complaint.
Notice of Release: If the FDA finds the product/s compliant after examination, the importer of record, consignee (when applicable) filer and CBP are notified by a Notice of Release that states the article may be admitted as far as FDA is concerned. However, the CBP or another PGA may still have pending issues that must be resolved. The pending issues with the CBP or another PGA may cause the delay.
Notice of Refusal of Admission: If the importer fails to meet the authorization for reconditioning, FDA issues a Notice of Refusal of Admission to the importer, consignee and when applicable, the filer with a copy to CBP.
Interactions with FDAs
- The NOA will explain what action the FDA has taken or will take. Pay close attention to the notice and ensure you understand what FDA is doing and which product or products are covered in the notice.
- Pay attention to each line on your invoice, the FDA's decision is made based on each item on the invoice.
- When speaking to the FDA staff, always have a copy of the NOA in hand.
- Highlight the information such as entry number, line item description handy.
The Process if the FDA sends a notice of sample:
Take a deep-dive into the New Import program for 2018 attend the 2-Day in-person Seminar. The seminar will help you correctly use new import procedures and programs. The speaker Casper (Cap) Uldriks brings over 32 years of experience from the FDA.






