The Design, Control, Monitoring and Validation of Water Systems for Pharmaceuticals, Biologics, Medical Devices, Cosmetics, and Personal Care Products
If you are a microbiologist or a professional at a site that manufactures drug products and drug substances, you must understand the microorganisms in water systems and how best to monitor and control them. Having a high purity water system alone does not guarantee contamination-free facility processes and products.

It is crucial to understand the microorganisms in water and learn how to apply ongoing microbial control to ensure proper system design, system sampling, system maintenance, and sanitization practices. It is also important to recognize the common problems of sanitization process, the causes of sanitization failures and how to troubleshoot and take remedial actions with respect to material, construction methods, biofilm removal process and timing of sanitization.
USP <1231> states "Microbes in water systems can be detected as exampled in this section or by methods adapted from <61> Microbial Enumeration Tests and <62> Tests for Specified Microorganisms or the current edition of Standard Methods for the Examination of Water and Wastewater by the American Public Health Association."
This article provides background and guidance for maintaining high purity water systems from a microbiological aspect:
- System design
- System validation
- Microbial limits
- Water for injection systems
- Still
- Heat exchangers
- Holding tank
- Pumps
- Piping
- Reverse osmosis
- Purified water systems
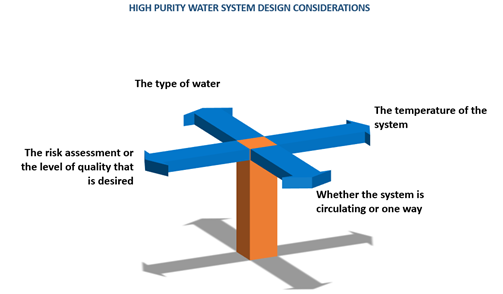
System design
- The type of water
- The temperature of the system
- Whether the system is circulating or one way
- The risk assessment or the quality that is desired
System validation
- Description of the system.
- Drawing showing all equipment in the system from the water feed to points of use and all sampling points and their designations
Microbial limits
- Sterile water is a requirement
- Less than 10 CFU/100ml is an acceptable action limit as per FDA
- All limits are action limits
- 100 - 300 mL is preferred sample size when sampling Water for Injection systems
- Monitoring of WFI systems for or both endotoxins and microorganisms is important because it can pass the LAL endotoxin test while failing in the above microbial limits
Stills
Heat exchangers
- To ensure that the higher pressure is on the clean fluid side, provide gauges for constantly monitor pressure differentials
- Utilize the double-tubesheet type of heat exchanger
- Perform periodic visual inspections to detect early signs of leaks in your tubesheet or gaskets
- Visual inspections also can help you detect early signs of fouling. It will help you determine whether you should make any changes to the process to prevent fouling.
Holding tank
Pumps
Piping
Reverse Osmosis (RO) and Deionizers
Purified water systems
The type of the product that is to be manufactured determines the design of the water system. Design considerations are shown in the following figure.

Hot systems are self-sanitizing. (65 - 80oC)
Circulating water is less likely to have high levels of contaminant, unlike one-way water.
As already discussed, different products require different quality waters. The microbial action limits should be determined after the evaluation of each product manufactured with the water from their system.
Learn how to design, validate and maintain the new and existing water systems used in the drug product manufacturing process in the webinar Pharmaceutical Water System: Design, Testing and Data Management.
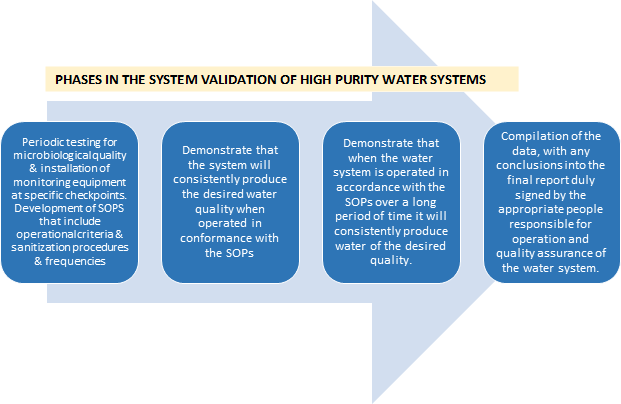
"Validation often involves the use of an appropriate challenge. In this situation, it would be undesirable to introduce microorganisms into an on-line system; therefore, reliance is placed on periodic testing for microbiological quality and on the installation of monitoring equipment at specific checkpoints to ensure that the total system is operating properly and continuously fulfilling its intended function." - Parenteral Drug Association Technical Report No. 4 titled, "Design Concepts for the Validation of a Water for Injection System."
Validation report must include:
Annually, a comparison should be made to the actual system to make sure that it is accurate, to identify unreported changes, and to approve reported changes to the system.
Water for injection systems (WFI)
Purified Water Systems
"The significance of microorganisms in non-sterile pharmaceutical products should be evaluated in terms of the use of the product, the nature of the product, and the potential harm to the user." (USP Microbiological Attributes Chapter <1111>)There are no clear microbiological specifications for purified water systems. However, it is left to the manufacturer to assess their product, its manufacturing process and establish an acceptable action limit of contamination and stay within the limits for the water system based on the highest risk product manufactured with water.
Water for injection systems
Feedwater should be pretreated for RO units and is recommended by most distillation equipment manufacturers. Seasonal variations and some other factors are outside the control of the pharmaceutical facility. The quality of the incoming feedwater may vary during the life of the system based on such factors.
By periodically monitoring the feedwater, the extremes can be determined. The design of the water system should be designed to operate inside the boundaries of the expected extremes.Stills should be properly validated to ensure that they are operating in a controlled state. Usually, the chemical quality is monitored using conductivity meters on water systems.
Two ways to prevent contamination by leakage:
Holding tanks must be verified for resistance to chemical sanitizers. It should be suitably insulated to store the water at a high temperature. To tolerate fluctuations in water levels and prevent collapse it should have a vent fitted with a hydrophobic air filter to prevent microbial contamination from outside air. The vent should in a position that is readily accessible.
Pumps should be assessed for proper function periodically. Any defective valves or seals must be promptly replaced. Also when pumps are not in continuous operation, water can lie in a static area. Care should be exercised to check such out.
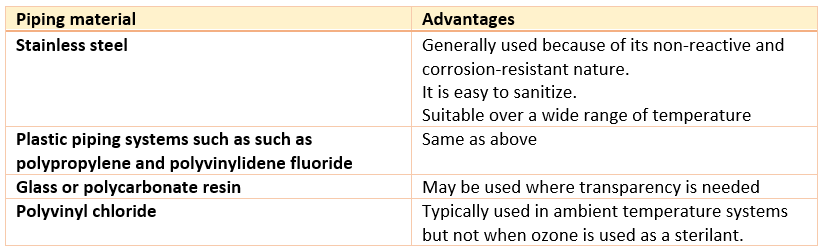
There should be sanitary fittings in all pipe joints or they should be butt-welded. Piping systems should be designed in such a way that sanitization, and thermal cycling. They must be specified for drain ability. Their design should be designed for reliability, pressure control and evasion of extractable contaminants.
RO remove dissolved solids from feedwater. Deionizers remove charged particles using ion exchange resins. To ensure the charged particles hold their ability, resins must be regenerated on a periodic basis using caustic and acid solutions. Either hydrochloric or sulphuric acid is used to regenerate cationic resins resulting in the replacement of the captured positive ions with hydrogen ions. Sodium or potassium hydroxide are generally used to regenerate Anionic resins resulting in the replacement of captured negative ions with hydroxide ions. An additionally integrated pretreatment cartridge pack with activated carbon, a 0.5B5 prefilter, and a calcium hardness sequestering compound is included in the RO pack. To minimize calcium carbonate precipitation and bind calcium ions, Sequestering agent which is a solid, long chain polyphosphate is used. The RO membrane is protected from damage that can be caused by fouling from particulates, chlorine oxidation, and formation of mineral scale on membrane surface by using the combination of pretreatment. To prevent bacterial growth, periodic chemical sanitization treatments must be performed.
The key is to ensure uniformity of temperature and maintain fast-flowing water. Most of the typical organisms will be killed at 65B0C (and above). However, there are chances of survival are higher should a biofilm community develop. When it comes to biofilm risk, Gram-negative bacteria form biofilms more readily than Gram-positive organisms. The reason for this is the extrapolymeric substances which are the basis of the biofilm 'slime', and lipopolysaccharides are found in abundance. Typically, Gram-negative bacteria are the most widely existent bacterial populations in the aquatic environments and hence this is of importance to water systems. So good design principles are the key to preventing bacterial growth aside from the efforts to maintain a temperature sufficiently high.
Attend the seminar The A to Z's of Microbial Control, Monitoring and Validation of Water Systems for Pharmaceuticals, Biologics, Medical Devices, Cosmetics, and Personal Care Products to gain a microbiology-focused education about all aspects of water systems and how biofilm manages to thrive there. The instructor will provide the necessary background needed to understand this very important subject matter. This understanding is essential for the proper design, validation, operation, monitoring, and maintenance of a high purity water system. Without this understanding, water system control and monitoring consists of a set of rules that often don't work or result in erroneous monitoring data and can cause everything from very costly and unnecessary system downtime to patient injury and product recalls. This 2-day course is particularly relevant to managers, supervisors, and operatives taking on new responsibilities related to water, but also for experienced water personnel to learn the "true" whys behind what they do and perhaps better ways of doing things.
The speaker T.C. Soli, Ph.D., is President of Soli Pharma Solutions, Inc, with training, auditing, and troubleshooting expertise covering water systems, sterilization, aseptic processing, contamination control, and microbiological laboratories. He has over 34 years of combined pharmaceutical experience as a consultant and with operating companies (DSM Pharmaceuticals, Glaxo Wellcome, Burroughs Wellcome, and Pfizer). Dr. Soli's career-long water system and manufacturing contamination troubleshooting expertise, coupled with water-related USP, ISPE, PhRMA, and PDA committee and guide creation involvements, afford him practical knowledge about process and contamination control and mitigation; cleaning, sterilization, process, and microbiological testing validation; and all aspects of high purity water systems.
Dr. Soli is in his third 5 year term on USP Expert Committees responsible for Pharmaceutical Water, previously served 5 years on the Advisory Panel to the USP Microbiology Subcommittee, and helped develop the Water Conductivity and TOC specifications used by USP and adopted world-wide. He has authored many articles and chapters in books and industry guides published by PDA and ISPE and is the author of USP's Chapter <1231>.






