GMP Inspections - What the Historic EU-US Mutual Recognition Agreement Means to Pharmaceutical Companies?
- Are you aware of the Historic EU-US mutual recognition agreement (MRA) for inspections of drug manufacturing facilities?
- Did you know that the MRA is fully implemented in their respective territories?
- How does that agreement impact your pharmaceutical company and your job?
Understanding the answers to the above is vital in passing a GMP inspection. This article will examine the answers.

What the EU-US Mutual Agreement is all about?
In July 2018, a Joint Statement was agreed by President Juncker and Trump. That included a commitment from the EU and the US to reduce barriers and increase trade in a range of sectors, including pharmaceuticals. A significant element of the Joint Statement was delivered with the implementation of the mutual recognition agreement (MRA) for inspections of sites when the last outstanding EU Member State recognized by the U.S. Food and Drug Administration (FDA) of Slovakia on 11 July 2019.
With this implementation, drug inspectors from the FDA and EU can use each other's inspection data during inspections in each other's country. While this agreement helps increase the efficiencies between the two agencies by avoiding redundant inspections, it also enables the agencies in focusing their resources on inspections of drug manufacturing sites in other countries. However, the agreement has implications for pharmaceutical companies as well.
The MRA applies to most human drugs and veterinary drugs with some exceptions. Those products not included are:
- Human blood
- Human plasma
- Human tissue and organs
- Veterinary immunologicals
What the EU-US Mutual Agreement means to drug companies?
Reduced Inspections at manufacturing facilities in the EU and US
Remember those days when you got to know that inspectors from FDA were going to inspect your Facility. Not long after the FDA inspection, inspectors from the European Medicines Agency (EMA) arrived for an inspection again. With the MRA signed, it's a sigh of relief to drug manufacturers. Many manufacturing facilities on both sides see fewer inspections. Hardly will companies see duplicate FDA and EMA and GMP Inspections.
Increased inspections at manufacturing facilities in other countries
Manufacturing Companies in countries outside of the EU and US see an increase in inspections post the US-EU MRA. This is because the EU and the US import a large proportion of the APIs and finished pharmaceuticals from countries outside the US. The agreement allows agencies at both sides to allocate their inspection resources towards facilities that supply these APIs and finished pharmaceuticals. Thereby, with increased inspections, adequate levels of controls are applied to enhance product quality and safety.
Reduced time to market batch products
Before the MRA, drug product categories listed above, that entered the US had to go through batch testing at a site in the EU to comply with the EU Marketing authorization. Now, this additional batch testing is not required if there is evidence that the batch testing was done in the US, the product was manufactured in the US and a certificate that confirms compliance of the product with the EU marketing authorization requirements. Thus, drug companies are benefitted with the simplified process of some imported products.
Improved communications and streamlined access
Post the MRA, drug manufacturers stand to benefit from the improved communication of actions that result in drug shortages, product-recalls, counterfeits, and defects.
Faster and less costly for both sides to bring medicines to the market
The EU and the US compliant companies are enabled faster access to mutual markets and other countries that depended on the inspections of the FDA and EMA.
Europe and the United States account for more than 80% of global sales of new medicines. However, a large proportion of the APIs and finished pharmaceuticals are imported from countries outside their territories. The agreement has enabled both FDA and EMA to increase inspections in countries which represent higher risks of noncompliance.
Higher cost of noncompliance
'Together, Europe and the United States account for more than 80% of global sales of new medicines.' ( European Commission). With inspections meeting the strict standards of the EU and the US together, there is an influence in the distribution of products sold in the two biggest markets. With sites coming under the jurisdiction of both markets, companies will face stringent enforcement actions if found noncompliant.
Conclusion
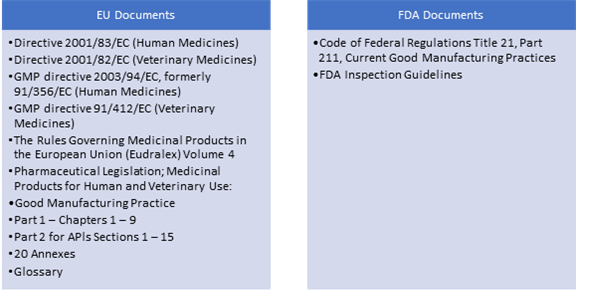
Inspections post the implementation of the MRA carry more weight because they are accepted by multiple agencies. So, drug manufacturers must gain a complete understanding of the major differences between EU and US regulations. Understanding the GMP regulations and inspections of the EU and the US is more crucial now than ever.
Supplementary Information
The following training programs related to the topic might interest you:
GMP auditor training for pharmaceutical companiesFood cGMP's 101: Understanding critical FDA expectations for safe food manufacturing to assure a favorable audit
Annual Current Good Manufacturing Practices (cGMP) Training
Current Good Manufacturing Practices (cGMPs)
A Holistic Approach to External GMP Surveillance, GMP Training and Quality Knowledge Management
Good Manufacturing Practices for Active Pharmaceutical Ingredients (APIs)
Creating FDA-compliant cGMP Training Program
Information Technology Change Control in cGMP regulated environments
PASSING A US FDA GMP INSPECTION: A-Z Guaranteeing a Pass the First Time through an FDA Inspection
The Regulatory Bodies enforcing GMP Requirements

Regulations and Guidance