Pharma Change Control Concepts
- Change control is needed to assure that changes do not impact
- Product Identity, Strength, Quality, Purity, Potency
- Validated Processes/Systems
- Regulatory Commitments (e.g., NDA / ANDA / BLA)
- To comply with cGMP requirements and guidance for change control.
- To provide a single, easy to access the source of information regarding changes for
- Audits/Inspections
- Annual product reviews (internal)
- Annual reports (to FDA)
- Manufacture a product that meets the specification
- Keep equipment free from failure
- Ensure sterility assurance
- Comply with cGMP requirements
- Prevent product quality problems including FDA 483 findings, warning letters, sub-standard products, or product recall.
Why is Understanding Change Control So Important for Pharmaceutical Manufacturers?
One of the top 10 FDA 483 and Warning Letter citations is for inadequate change control. Change control receives detailed scrutiny during FDA inspections, and FDA reviews change control documentation to determine that changes did not adversely impact products, processes, equipment, facilities, etc. A single inadequate change may lead to significant negative events, including the release of a sub-standard product or product recall. A pattern of inadequate changes may require costly and time-consuming system remediation efforts.

Change control is a complex but very important aspect of GMP compliance.
Understanding the Basics
Before manufacturing a drug, a company must seek approval from the relevant federal agency to ensure that the drug is compliant with the required standards for quality, efficacy and safety.
Also, there are several other requirements to follow the Good Manufacturing Processes (GMP). Companies must document standard operating procedures for manufacture and quality control. They must clearly define the prerequisite conditions and materials such as trained personnel, suitable rooms, documentation types required for reproducible quality.
Before implementing these requirements, they need to be reviewed by a regulatory body for suitability for the intended use. In this approval model, the regulatory bodies conduct the review as part of an authorization procedure and send a notice of approval to the applicant that the product is authorized for use.
Manufacturing companies must provide evidence of the suitability of the equipment/facilities and procedures with qualification/validation. In such cases, the qualification/validation report confirming suitability and authorization for use must be signed by a responsible person.
Compliance with the suitable requirements is needed not only the first time that a drug is manufactured or a procedure is followed at a facility but also throughout the entire history of a drug or a procedure. Just as how companies are required to document the complete history of the batch, they are also required to have written specifications for materials or directions for procedures. Each change control must be documented.
What is Change Control?
A company may need to make changes to a process or procedure for reasons including changes to fundamental legal requirements, scientific/technical development, business constraints, or other reasons. They may have to redefine, enhance, amend or cancel requirements over and over again. Whenever a change is made to a process or procedure, it is reported by change control procedure and that is approved by the company authority.
Annex 15 of the EU GMP Guidelines defines "change controlb" as: "A formal system by which qualified representatives of appropriate disciplines review proposed or actual changes that might affect the validated status of facilities, systems, equipment or processes. The intent is to determine the need for action that would ensure and document that the system is maintained in a validated state."
Handling Change Control
Regarding the handling of change control, Chapter 5.23 of the EU GMP Guidelines states the following:
"Significant amendments to the manufacturing process, including any change in equipment or materials, which may affect product quality and/or the reproducibility of the process should be validated."
Also, the Code of Federal Regulations (CFR) provides brief notes on the topic of "change control":
§ 211.100 Written procedures; deviations
(a) "There shall be written procedures for production and process control designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess. Such procedures shall include all requirements in this subpart. These written procedures, including any changes, shall be drafted, reviewed, and approved by the appropriate organizational units and reviewed and approved by the quality control unit."
§ 211.160 General requirements. (a) "The establishment of any specifications, standards, sampling plans, test procedures, or other laboratory control mechanisms required by this subpart, including any change in such specifications, standards, sampling plans, test procedures, or other laboratory control mechanisms, shall be drafted by the appropriate organizational unit and reviewed and approved by the quality control unit. The requirements in this subpart shall be followed and shall be documented at the time of performance. Any deviation from the written specifications, standards, sampling plans, test procedures, or other laboratory control mechanisms shall be recorded and justified."
As change control covers a wide area of application, it is the task of the entire company. It's not the task of a single department or team.
"Written procedures should be in place to describe the actions to be taken if a change is proposed to a starting material, product component, process equipment, process environment (or site), method of production or testing or any other change that may affect product quality or reproducibility of the process. Change control procedures should ensure that sufficient supporting data are generated to demonstrate that the revised process will result in a product of the desired quality, consistent with the approved specifications. "(Annex 15, no. 43)
"Change control is an important element in any Quality Assurance system. Written procedures should be in place to describe the actions to be taken if a change is proposed to a product component, process equipment, process environment (or site), method of production or testing or any other change that may affect product quality or support system operation." (PIC/S document PI 006, section 6.7.1).
Why is Change Control Necessary?
Change control helps keep up with the 5 values.
Safety:
Change control helps ensure that the product is free of side effects when it is used by the consumer.
Identity:
The product is exactly what the label and associated materials say it is.
Strength:
The product delivers the right dosage and strength over its shelf-life.
Purity:
The product is free from physical, biological, and chemical contamination.
Quality
Manufacturers establish and follow quality systems to help ensure that their products consistently meet applicable requirements and specifications
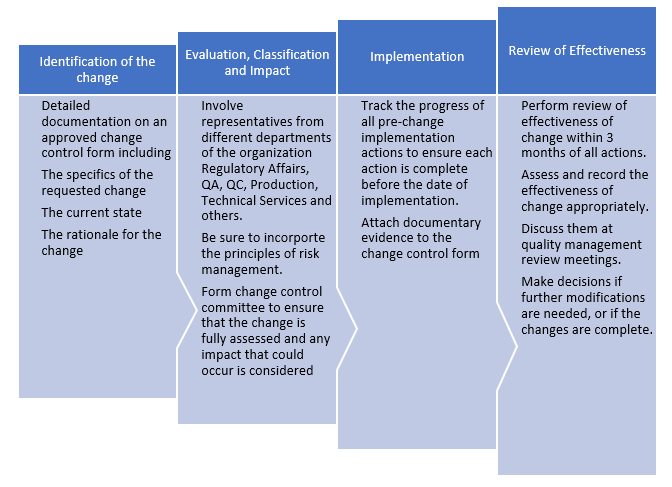
Change Control helps achieve the following:
Steps to Avoid the Unintended Consequences of Change
Thus, change control is a quality system. It is not reactive. It is willful and deliberate. It's just not about completing a form, revising a document, generating or executing a work order. It involves much more than validation activities, project management, or logistics.
Ultimately, what has changed, why the change was made, what risks were identified and/or mitigated, how the change was made, and whether the change was effective must be clearly understood.
Change Control Seminars and Webinars
Change Control Best Practices: Avoiding the Unintended Consequences of Changes
This is a practical how-to course, designed to provide participants with skills they can immediately apply to change controls within their own organizations. Case studies will allow participants to practice skill sets in cooperation with the instructor.
Effective Systems for Change Control in the Pharmaceutical Industry
This webinar will help you understand GMP requirements for change control from a pharmaceutical manufacturing perspective, the purpose of change control and what types of changes are or are not subject to change control. Participants will learn how to execute and implement a change in a regulated environment.