Improving Processes and Systems in A Pharmaceutical Quality Control Department
As a person in a quality leadership role, you don't want to leave any stone unturned to improve quality. You aim to achieve excellence while ensuring speedy delivery of the product for use
This article intends to take a look at the quality control processes, systems, and recommend appropriate ways to close the gaps and make continuous improvement.
Although the quality management system has many facets in its classification, in this article we explore a subset of the QMS which is Processes and Systems. We begin with how the definition of quality has evolved and moves on to examine the typical activities in a quality control department to formulate a plan to improve processes and systems.

- Regulatory compliance
- Risk management
- Transparency & accountability
- Conformance
- Processes & approach
- Audit & mentoring
- Business continuity
- Policies & procedures
- Reporting
- Inventory management
- Certificate of analysis
- Performance management
- Competency & capabilities
- Leadership development
- Case management
- Workflow management
- Planning
- Continuous improvement
- Testing
- Product testing
- Use of statistics
- Resource control
- Complaints management
- Focus on process and documentation
- Assurance standards and quality management
- Quality assurance programs
- Compliance with evolving regulatory standards
- Focus on quality and process manuals
- QA checks for tools and software
- Compliance with ISO standards
- Prioritizing customer satisfaction Strategic planning
- Change management (CM)
- Continuous process management (CPM)
- Customer experience and impact of society
- Adherence to evolving customer requirements
- Managing Quality control tests
- Managing Quality control specialists and their workload
- Allocating and calibrating test instruments
- Establishing appropriate test methods
- Documenting test specifications
- Performing tests in the sequence of priority
- Accurately recording test results
- Capturing quality data for understanding the reliability of the manufactured batches
- Trending and analysis
- Stability studies
- Quality management
- Compliance with the evolving challenges
- Take control of documentation such as Certificate of Analysis (CoA)
- Safety
- Corrective and Preventive Actions management (CAPA)
- Location directives
- Raise flags when two reactive chemicals are placed in proximity
- User Adaptability and training to conform to the new Quality standards
- Efficient Document Management
- Addressing Nonconformance through a unified platform to log non- conformance, complaints, quality of incoming raw materials, delay, etc.
- Addressing CAPA using advanced analytical features to help learn and unlearn from past records
- Tools and technology for process automation, data entry error reduction, and tracking process efficiency
Quality Management System Classification
| QUALITY MANAGEMENT | |||
|---|---|---|---|
| CORPORATE GOVERNANCE | PROCESSES & SYSTEMS | LEADERSHIP & SUPERVISION | REDESIGN & IMPROVEMENT |
|
|
|
|
|
The definition of quality management has been evolving with time. The US FDA is becoming more stringent with its norms to ensure higher quality standards, and companies are responding to such challenges by raising their bar to meet the quality standards. The following table depicts how quality has evolved since 1970.
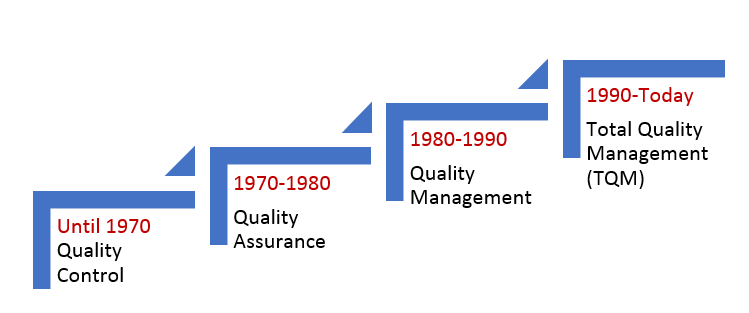
|
|
|
|
|
|
|
|
|
|
|
Improving Quality Control Processes
Your department uses dozens of processes every day. If they are dysfunctional or inefficient, they could cause problems including unhappy customers, stressed colleagues, missed deadlines, increased cost, etc. The first step to improving quality processes is to review the activities performed in the department. Following are the activities a typical quality control team performs.
Your Quality Department's day could generally start with lab analysis and paper requests to conduct sample tests on a sample batch size.
It could be just a few or many batches for products received from third-party manufacturers or those produced in-house.
Your quality lab prepares test instruments for each test and accurately records the results of the test and obtains digital signs-off for every work order.
When you take a close look at what it takes to do it right, your perspective on all of the necessary requirements for an ideal process can shift.
Your department keeps all the SOPs open in front of them to perform a test with accuracy and not allow any deviation in the process unless the "method to deviate" is clearly stated.
Only if there is a robust system is in place, your department will find it easy to comply with a process. If it is a paper-based system, the process will be complex with corrections and piled-up paperwork.
It would be cumbersome to dig through piles of papers for the correct answers during an audit. A central filing system to manage all the documents can help ease the tasks and greatly improve the process.
Quality Control Systems
If you're putting an electronic system in place, you'll need to consider how robust the system needs to be while also ensuring it is compliant and user-friendly. With more controls, the process could add discomfort to users.
Since any change will have a digital stamp linked back to the person who modified the data, it could make people more anxious of making mistakes. It may also be time-consuming to correct errors in retrospect while using a controlled and validated system that complies with 21 CFR part 11.
The goal isn't to make users feel uncomfortable, but to help them develop the right habits. Ideally, business users should be given sufficient details about the overall system's operation. A well-designed training program will assist users to become more acquainted with the system - fostering the process within the company.
Anybody with a fundamental understanding of compliance would prefer an electronic system over a paper-based process, for its capability to capture data as is in its current state.
It then necessitates a change in attitude from "constantly fixing mistakes" to "proactively doing it right the first time." Your ultimate goal is to streamline tasks to improve efficiency throughout the organization. You can achieve this goal by evaluating a dependable method that your department can easily adapt.
Quality Management Challenges and How they are Addressed
| Challenges | How they are Addressed |
|---|---|
|
|
|






