Preventing Human Errors by Crafting Effective SOPs
'To err is human.' Many business leaders with loads of experience acknowledge that human error is a major cause of quality and production losses. Although errors cannot be fully eliminated, they can be prevented by following certain sets of procedures called as "Standard Operating Procedures" (SOP). If you are a regulatory professional responsible for process improvement in your organization, it is crucial to understand how to create or revise SOPs in a controlled and efficient manner. While well-written SOPs help in maintaining compliance and delivering quality products, poorly written SOPs can quietly grow into a host of significant compliance issues.

Why SOPs?

In the ever-evolving regulatory environment, leading pharmaceutical and medical device companies have embraced the creation SOPs as a means to improve quality and save significant costs. While SOPs are created to improve the company's efficiency and effectiveness, they are also a regulatory requirement. For example 21 CFR 211.100, the regulation states:
Also, the FDA requires all pharmaceutical and medical devices companies to set up and operate a quality management system (QMS) compliant with CFR Part 820. This requires Standard Operating Procedures (SOPs) to be created for all systems and operations that impact the quality and safety of drugs and medical devices.
SOPS are created to inform individuals in an organization what is expected of them, what are the boundaries they can operate in, what defines success and more. The following points out to the specific purpose of writing SOPs.
- To define the tasks of individuals
- To train staff about a process
- To create consistency in the way an individual carries out the defined task or activity
- To provide operational staff with all the safety, health, environmental and operational information required to perform a job properly
- To protect the health and safety of staff, and to protect the environment
- To protect the community
- To ensure that processes work smoothly and follow the prescribed timeline
- To protect the staff or anyone around the community from harm and ensure successful processes
- To communicate process changes to staff
- To ensure compliance with the regulatory and company procedures and processes
- To serve as a chronological record of the how, why and when of steps in a process for use are made, when they were modified, and when the SOPs must be revised
- To serve as a description of steps in a process so they can be reviewed in accident investigations
- To serve as a checklist for co-workers who observe job performance to reinforce proper performance
- To serve as a checklist for auditors
How to write effective SOPs?
Craft procedures from the perspective of the end-user
While crafting the SOP, It is important to understand the point-of-view of the user of the SOP. The following tips will help you write SOPs for users:
Write briefly, clearly, and follow a step-by-step easy-to-read format
Write short and crisp sentences using simple and common terms. Don't replace simple, clear words or phrases with complicated, clichC)d or jargon-filled terminology
Use active voice and deliver the key idea first
The writing center of the University of Wisconsin-Madison rightly states 'Passive voice sentences often use more words, can be vague, and can lead to a tangle of prepositional phrases. 'On the other hand, SOPs that use active voice emphasize action rather than the actor, keeps the subject and focus consistently throughout a passage, and creates an authoritative tone.
Don't be vague
Avoid generalized terminology. Be specific. Convey information in explicitly. Leave no doubt as to what is required. When needed use a flowchart to demonstrate the process that is described.
Use smart formatting
Use the style guide used by your organization. Maintain consistency in font size and margins across SOPs. If the SOP is long and has dense paragraphs, use bulleted items and lists to capture and hold the attention and slow the reading pace of the user.
The SOP process
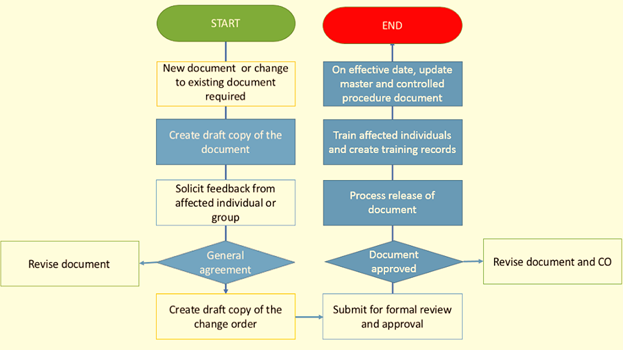
A general format as an SOP model
HEADER
- Title identifying the activity in question with appropriate keywords
- Document number
- Version
1.0 PURPOSE
- Define the Intent of the document in not less than one or two sentences clearly stating what the document contains
2.0 SCOPE
- Define who the particular set of procedures are for
- Define what is in scope and what is not
- If interpret if there is anything that needs to be
3.0 REFERENCES
- Provide reference to other documents needed to understand and effectively execute the procedure
- These may include
- Other SOPs
- Government-issued documents
- Title and identifying numbers for the referenced documents
- Or entire reference cited using the standard reference format for publishing
4.0 DEFINITIONS
- Define terms not familiar to end users
- Spell our acronyms or abbreviations used
5.0 ROLES AND RESPONSIBILITIES
- Define roles and responsibilities for executing tasks within the procedure
- Narrow down the scope if required and create many SOPs to complete the task
6.0 PROCEDURE
To ensure that you meet the FDA standards and don't complicate the writing, break it down into the following components:
- Key phases
- Individual action items within each key phase
- Separate notes clarifying the process and/or responsibilities and potential cautions
7.0 APPENDICES
- Preferably in the form of flow charts
8.0 HISTORY OF THE REVISIONS
- Document the changes made to a procedure providing justification for why the procedure was created
APPROVAL SIGNATURES
Key roles generally include
- Author
- Reviewer
- Management approver
- Quality reviewer/approver
The 21 CFR part 11 requires the Quality control unit to approve all procedures that may impact the "the identity, strength, quality, and purity of the drug product."
A clearly written SOP can aid regulators to grasp your procedure without requiring to probe in too much during an inspection.
Attend the webinar 'How to write SOP's that Avoid Human Error' to understand human behavior and the psychology of error as well as identify exactly where the instructions' weaknesses are, so procedures can be human engineered, improved and/or fixed. This webinar offers practical approaches to address writing rules to reduce the likelihood of procedures.
The instructor, Ginette Collazo, PH.D, is a human error and human behavior expert. She has spent more than 15 years in technical training, organizational development and human reliability areas. She has worked with Bristol-Myers Squibb, Johnson & Johnson, Schering-Plough, Wyeth, and has been a consultant with major firms like Abbott, Johnson & Johnson, Perrigo, among many others. She has also implemented human error reduction programs and technology in many small and mid-sized drug and device companies. An active researcher in specialized studies related to human reliability, she is the author of numerous publications on these topics.






