Understanding the Regulatory Standards for Radiation Sterilization of Medical Products
Medical products must be sterilized prior to their use. Radiation sterilization deactivates microorganisms such as bacteria, fungi, viruses, and spores by relying on ionizing radiation, primarily gamma, X-ray or electron radiation.
The medical device, biologic products, and pharmaceutical professionals who desire to get a complete understanding of the validation and use of radiation sterilization for their products will find the following information useful.

The regulatory standards establish certain criteria that should be met prior to the production of medical products.
What medical products are required to be sterilized?
- Medical disposables
- Tissue and bone implants (including substitutes)
- Pharmaceuticals and cosmetics (information largely with relevant companies)
Examples include sutures, surgical gloves, gowns, face masks, syringes, sticking plasters, dressings, and other single-use healthcare supplies.
What are the regulatory standards and requirements for radiation sterilization of medical devices?
North America:
- AMI ST 32 "Guideline for Gamma Radiation Sterilization"
- AAMI ST 31: AAMI ST 31 "Guideline for Electron Beam Radiation Sterilization of Medical Devices
International
- ISO 11137 "Sterilization of health care products - requirements for validation and routine control - radiation sterilization"
- ISO 9001: ISO 9002; ISO 9004
Europe
- BS EN 552 "Sterilization of medical devices - validation and routine control of sterilization by irradiation" (EN 556; EN 1174-1; EN 46001; EN 46002)
The document ANSI/AAMI/ISO 11137-1, Sterilization of health care products-Radiation-Part 1: Requirements for development, validation, and routine control of a sterilization process for medical devices, incorporates methodologies of both ISO (International Standards Organization) and ANSI (American National Standards Institute)
Prior to the production of a sterile medical product, what predetermined criteria for validation should be met?
- Materials used in the product and its packaging should be evaluated
- The product's radiation stability should be determined
- The dose for sterilization should be selected
- Product dose mapping should be performed
- The process should be certified
- The sterilization dose should be audited
Documentation should be maintained throughout in order to demonstrate the viability and reproductively of the process.
Materials used in the product and its packaging should be evaluated
Radiation generally affects the materials used in the products, its components and packaging. It is therefore important to consider its effects before using the Gamma or electron beam radiation for sterilization of medical products.
Ionizing radiation creates different reaction on different polymers. In order to ensure that the sterilization process does not adversely affect the quality, safety or performance of the product throughout its lifecycle, it is crucial to verify the maximum dose likely to be administered during the sterilization process.

- The ANSI/AAMI/ ISO 11137-1994 document sets forth the physical and functional methods for evaluating the plastic materials.
- The product engineering or research staff can also perform tests that closely ballpark the actual mechanical application of the product.
- In order to demonstrate that the product claims and specifications have been met, the evaluation and test results should be maintained in the product's device history file.
- The manufacturer should establish a maximum permissible dose to ensure the prevention of adverse effects from high levels of radiation.
The dose for sterilization should be selected
The basis for the selection of the dose depends on the bioburden of the product and the maximum dose at which the product can retain its form, fit and functionality.
With increasing dosages, the possibilities of one organism surviving after the irradiation decline logarithmically. Nonetheless, the characteristics of the microbial population that describe a product's pre-sterilization bioburden should be considered.
Product dose mapping should be performed
The purpose of dose mapping study is to find the minimum and maximum dose zones within the product load using a preset pattern of loading. The study helps in verifying that the minimum sterilization dose is attained, the integrity of the material is maintained by not going beyond the maximum dosage limit. The reproducibility of the sterilization process is established using the dose mapping study. The dose monitoring locations for routine processing are selected based on the dose mapping study. Dose mapping should be repeated after any intervention such as service, repair, re-calibration that could change the settings.
The process should be certified
The certification process includes 3 steps
- Documentation
- Review polymer
- Approval
The certification is maintained in the product's device history file.
The sterilization dose should be audited
Initially, the dose determination is based on the natural bioburden of the product and packaging. However, the microbial population can change during the manufacturing process. The dose auditing is performed to ensure the validity of the dose despite the change in manufacturing process. Even if no change is made in the manufacturing process, the dose auditing should be carried out within 12 months.
Product/process validation
Regardless of where the radiation sterilization is performed in-house or with a contract sterilizer, understanding the process validation and process control requirements associated with the radiation process is vital to the product manufacturers. The following illustrates the same.
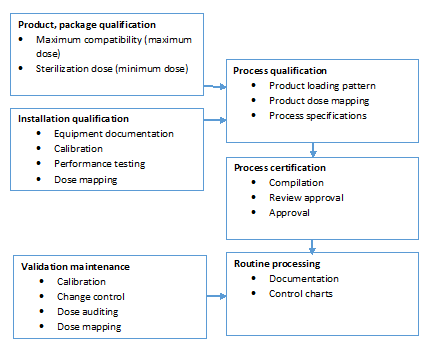
Attend the seminar 'Radiation Sterilization of Medical Products - Beyond the Basics' to take a deep dive into all the aspects of radiation sterilization validation, materials selection and processing implementation. This workshop has been designed to help attendees learn the ins and outs of all the radiation modalities, materials selection, and validation of the sterilization process per ISO 11137. Additionally, 483 case studies will explore how to avoid the operational and legal issues that arise from nonconformance with regulators (FDA) and auditors.
The speaker Gerry O'Dell, is owner and President of Gerry O'Dell Consulting, a consulting firm based in the United States with medical device and pharmaceutical clients around the world. Prior to starting Gerry O'Dell Consulting, she worked for Johnson & Johnson Medical, Inc. as the Manager of Laboratory & Sterilization Services which managed the sterilization program for J&J Medical in addition to providing laboratory testing services to several J&J companies. She holds both a Bachelor and Master of Science degree in Microbiology from the University of South Florida and has over twenty-nine years of experience in the medical device industry.






