ISO/IEC 27000 Checklist Kit
Author: Andy Coster CCP and John Neorr
Cover: Available
Format: Word® (To save money, click here for our PDF version)
Language: English
Page count: 800+
Provider: SEPT
Shipping: Available for download - Link will be provided in My ComplianceOnline section
Overview of the base standard ISO/IEC 27018:2014
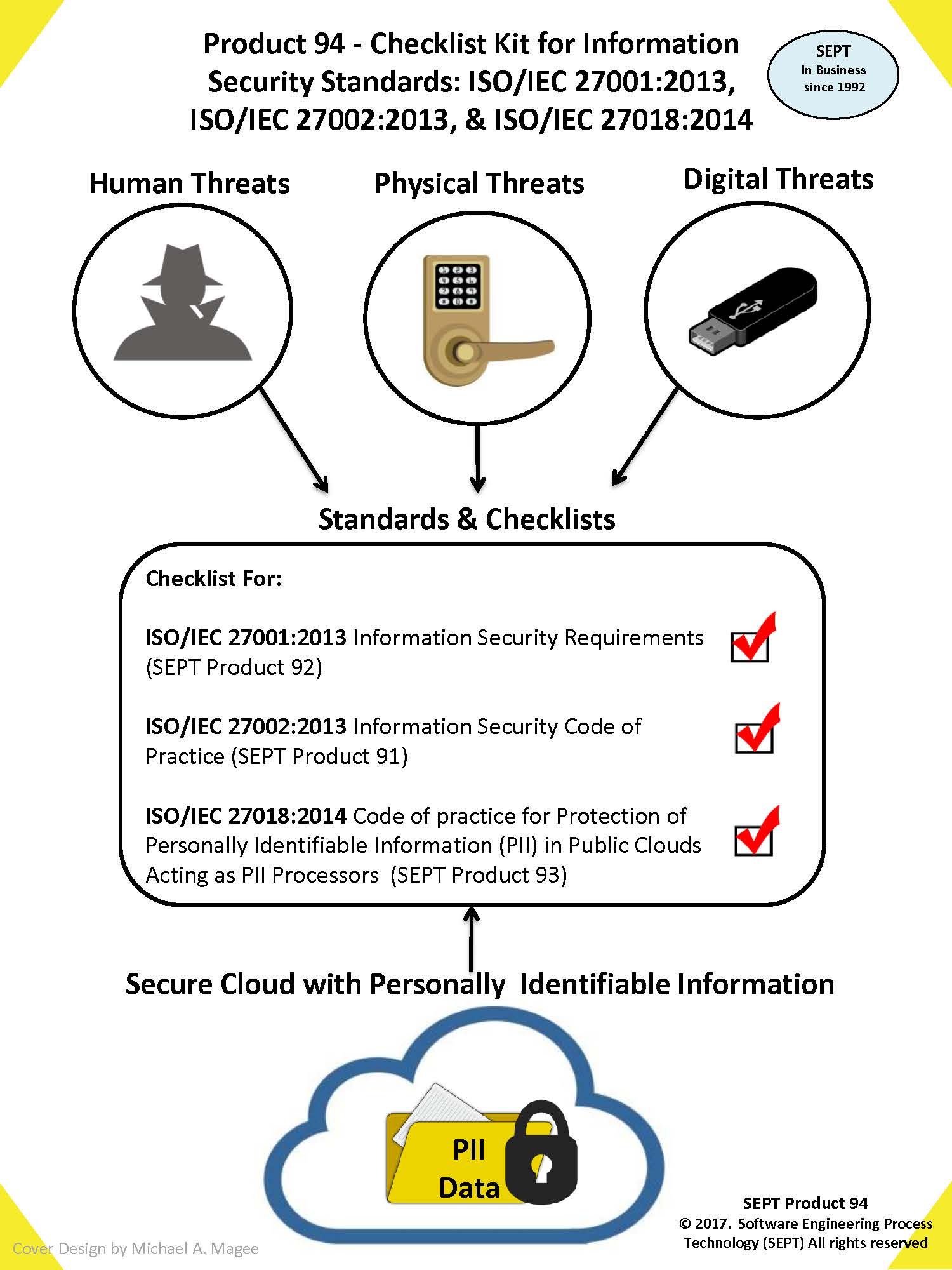
SEPT has packaged 3 of the key ISO/IEC 27000key checklists together in a kit. This is an opportunity for those firms getting certified to ISO/ IEC 270xx that need all the checklists to buy a package at a reduced cost. The kit is available in either PDF or Word Format - the latter format allowing you to easily tailor the documents to your specific organization and/or project needs.
The kit comes with 32 hours of free consultation from experts that have firsthand knowledge of the underlying standards and processes to which the documents refer. This offer is valid for 60 days after purchase of the product. Specific SEPT checklist products (documents) included in this kit are:
- Checklist for Standard ISO/IEC 27001:2013 Information Security Requirements
- Checklist for Standard ISO/IEC 27002:2013 Information Security Code of Practice
- Checklist for Standard ISO/IEC 27018:2014 (Code of practice for protection of personally identifiable information (PII) in public clouds acting as PII processors)
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- System Documentation Management Plan Template- Second Edition
Price: $330 BUY NOW - Template for a Software Maintenance Plan - Fifth Edition
Price: $330 BUY NOW - Validation Master Plan Item Information Form (RiskVal)
Price: $99 BUY NOW - Software Documentation Management Plan Template - Second Edition
Price: $330 BUY NOW - Templates and Plans for Software Configuration Management Documents-Version 6.0
Price: $330 BUY NOW - A Software Engineering Kit – Composed of Templates for Key Software Engineering Process Plans
Price: $660 BUY NOW