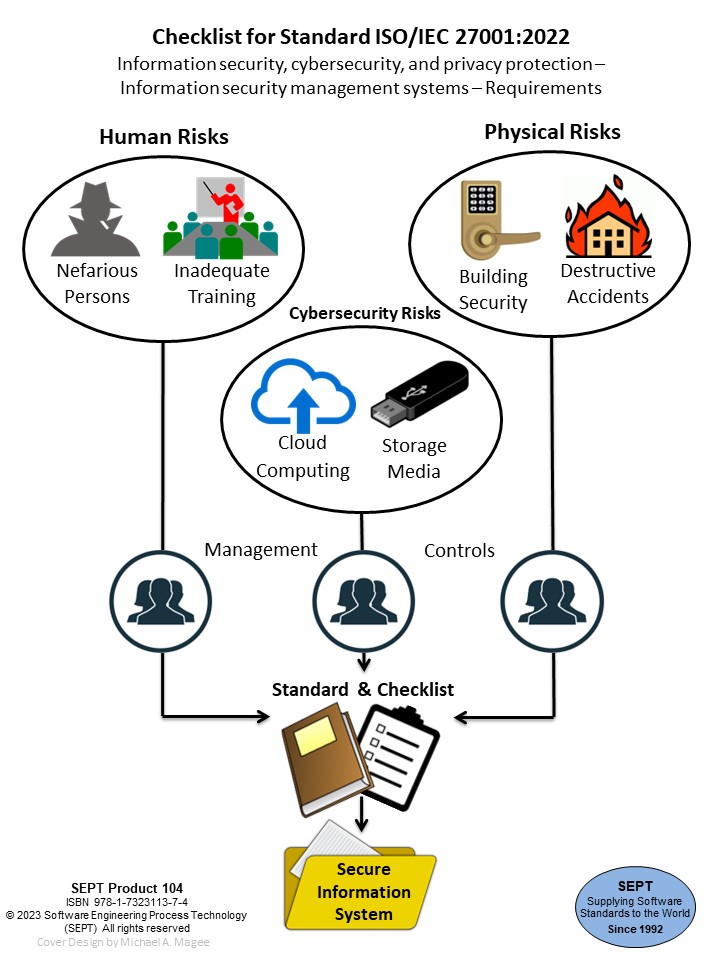
Checklist for Standard ISO/IEC 27001:2022 Information Security, Cybersecurity And Privacy Protection - Information Security Management Systems - Requirements
Author: Andy Coster CQI (Ret.) and Stan Magee CCP (Ret.)
Cover: Available
Format: PDF (Click here for our easy-to-modify Word® formatted version)
ISBN numbers: 978-1-7323113-7-4
Language: English
Page count: 124
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
ISO/IEC 27001:2022 provides requirements for organizational information security management system and information security management controls; taking into consideration the organization's information security risk environment(s).
It is designed to be used by organizations that intend to:
- Seek certification to ISO/IEC 27001:2022
- Select controls within the process of implementing an Information Security Management System based on ISO/IEC 27001:2022
- Implement commonly accepted information security controls
- Develop their own information security management system
The requirements included in the ISO/IEC 27001:2022 standard are listed at a high level of detail, with an Annexed reference to ISO/IEC 27002:2022 as appropriate guidance to demonstrate compliance with ISO/IEC 27001:2022. If an organization is interested in testing their compliance with ISO/IEC 27001:2022 this checklist will provide an analysis of the detail in the ISO/IEC 27001 standard. However, if the organization is only interested in the guidance in ISO/IEC 27002:2022 this checklist provides a list of all items required in Annex A of ISO/IEC 27001 that are derived from the ISO/IEC 27002 guidelines. They are described in the Introduction to the checklist and in section 9.
Customers of this product:
- ASTRONAUTICS CORPORATION OF AM
- BRIS, China
- DAIMLER AG
- Edpaudit, Nigeria
- HARGROVE ENGINEERS
- MED Institute, Inc.
- SIA, UK
- TPI, Aba Dubai
- UNICONNECT LC
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- ISO/IEC/IEE Standard 15288:2015-Systems and Software Engineering-System Life Cycle Processes - (Third Edition)
Price: $167 BUY NOW - Checklist for Standard ISO/IEC 27002:2013 - Information Security Code of Practice
Price: $167 BUY NOW - Template for a Software Maintenance Plan - Fifth Edition
Price: $149 BUY NOW - Evidence Product Checklist for ISO/IEC 12207:2017 ''System and Software Engineering - Software Life Cycle Processes''
Price: $167 BUY NOW - Templates and Plans for Software Configuration Management Documents-Version 6.0
Price: $149 BUY NOW - Software Documentation Management Plan Template - Second Edition
Price: $149 BUY NOW