By using this site you agree to our use of cookies. Please refer to our privacy policy for more information. Close
eCTD Issues for ANDA Submissions
- By: Rajesh Yelugoila
- Date: February 03, 2016
- Source: http://www.regulatoryone.com/2016/01/ectd-issues-for-anda-submissions.html
Compliance Webinars | Virtual Seminars for Professionals
Republished from www.regulatoryone.com with kind permission of the author.
eCTD Issues for ANDA Submissions
Post the implementation of GDUFA, in the current year 4 cohort of FY 2016, there has been a very significant positive change with respect to ANDA review timelines. The initial screening deficiencies/ information requests or acceptance letters are generally being issued within 1 month of filing ANDAs. The most important screening deficiency/information request issued by USFDA is related to eCTD aspects, which in general is given least importance. RA professionals generally ensure to meet the requirements of ANDA Filing Checklist, which doesn’t summarize the eCTD requirements in detail, but gives a link to USFDA website where we get all the guidelines and eCTD requirements details.
In this post, the below listed eCTD aspects will be discussed in detail, as they are important for avoiding eCTD related deficiencies.
- PDF Document Properties
- Fonts and Font Sizes
- Embedding of Non –Standard Fonts
- Bookmarks and Hyper Linking
- Requirements of Scanned Documents
- Leaf Title Naming (for eCTD XML)
PDF Document Properties
The file name is to be given in abbreviated form with the use of small letters.”Hyphens (-)” should be used in place of spaces.”Full Stops (.)”, should not be used.
- The title is generally not given under document properties.
- The acceptable PDF Versions are 1.4 to 1.7. Irrespective of the version of Adobe Acrobat, PDF Version should be anything from 1.4 to 1.7 only.
- There should not be any security passwords for opening the document
- “Fast Web View”, should be yes. If “Fast Web View” option is being displayed as “no” it has to be changed to “Yes” as per following procedure - go to “File” option in the menu bar of PDF File, go to “Save As Other”, then “Reduced Size PDF”. Under “Make Compatible with” option “Acrobat 5.0 and later” or “Acrobat 6.0 and later” should ideally be selected. Next “Ok” should be selected. Finally, the file in which this correction is being done, should be replaced (We get a message – “The file already exists. Replace existing file”. We should select “Yes” option). After this procedure, we should ensure PDF version is anything from 1.4 to 1.7 only.
- For Submissions to USFDA, Pages size should be 8.50 x 11.00 in.
- Under Initial View tab - Navigation tab should be set to “page only”, if the numbers of pages are less than 5 and there are no bookmarks. Any PDF file with more than 5 pages should be bookmarked in which case; navigation tab should be set to “Bookmarks Panel and Page”. Page Layout and Magnification should be set to “Default”.
- The maximum length of the filename should be 64 characters.
- The leaf element path length should not exceed 230 characters.
Fonts and Font Sizes
- Times New Roman 12-point font, is recommended and should be preferred for narrative text.
- Point sizes 9-10 are recommended for tables; smaller point sizes should be avoided.
- Point Size 10 is recommended for footnotes.
Embedding of Non –Standard Fonts
- All the non – standard fonts should be embedded. PDF viewing software, substitutes non-standard fonts if the font used to create the text is unavailable on the reviewer’s computer.
- In some cases, substitution of standard fonts like Helvetica or Times is done by PDF viewing software.
- Font substitution can affect a document’s appearance and structure, and in some cases it can affect the information conveyed by a document. Hence, embedding of fonts should be ensured.
- However, embedding of font should be avoided for labels and electronically signed documents.
The table with list of standard fonts are provided below.

Image Courtesy: Portable document format specifications guidance of USFDA
Note: There are multiple procedures for “embedding font”. You could Google it and follow. If you are facing any difficulty, I’ll be happy to help out.
Bookmarks and Hyper Linking
- There should be appropriate number of bookmarks in the document. The number of bookmarks should be neither less nor more.
- The number of sub heading trees within book marks should be limited to 3 or 4.
- If there is table of contents in the document, the bookmarks should exactly match with the table of contents (naming and number of bookmarks).
- The trick for giving bookmarks using Adobe Acrobat – Go to the page where bookmark is to be given, select the hand tool and go exactly to the title where the bookmark is to be given (it is to ensured that, no text is visible above bookmark title) and select the text, press control + B, the title automatically appears in the bookmark panel (if there are errors in bookmark title due to misreading of software, correct it manually), right click on title and press set destination, finally click on all the bookmarks and check whether they are going to exact destination.
- Hyper linking of texts to the destination is to be ensured, wherever applicable.
Requirements of Scanned Documents
- It commonly happens that, clarity of documents is not checked before submitting to the Agency. If we are not able to read a document due to lack of clarity, we definitely cannot expect the reviewer to read the document.
- The probability of Plant Layouts lacking clarity is more. Hence, clearly scanned Plant Layouts should be submitted.
- Please refer the below table for scanning resolution requirements
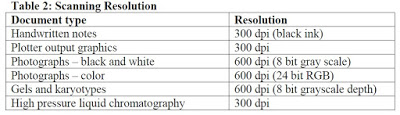
Image Courtesy: Portable document format specifications guidance of USFDA
Leaf Title Naming (for eCTD XML)
- The Leaf Title should be elaborate, considering ICH M4, M4Q guidelines, without the use of hyphens (-), full stops (.).
- Abbreviations should not be used.
- Examples of Correct and Incorrect Naming of eCTD Leaf Titles are provided below –Correct Naming – Description and Composition of the Drug Product Incorrect Naming – desc-comp-dp
Original source: http://www.regulatoryone.com/2016/01/ectd-issues-for-anda-submissions.html
About the Author:
Rajesh Yelugoila is a Analyst - Global Regulatory Affairs at Dr. Reddy's Laboratories where he is involved in the compilation of ANDA, ANDS and MAA for Injectable Drug Products in US, Canada and Europe respectively. He runs the http://www.regulatoryone.com site.
Disclaimer: The views expressed in this article are the author's own and do not necessarily reflect ComplianceOnline's editorial policy.
Trending Compliance Trainings

By - Roger Cowan
On Demand Access Anytime

By - Doug Keipper
On Demand Access Anytime

By - Joy McElroy
On Demand Access Anytime

By - Carolyn Troiano
On Demand Access Anytime

By - Dr. Afsaneh Motamed Khorasani
On Demand Access Anytime



By - Michael Ferrante
On Demand Access Anytime


- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
-
By: Miles HutchinsonAdd to CartPrice: $249
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
-
Add to CartSan Francisco, CA | Aug 6-7, 2020
-
Add to CartVirtual Seminar | Jul 16-17, 2020
-
Add to CartVirtual Seminar | Jun 18-19, 2020
-
Add to CartLos Angeles, CA | Aug 20-21, 2020
-
Add to CartVirtual Seminar | Jul 16-17, 2020
-
Add to CartVirtual Seminar | Jun 25-26, 2020
-
Add to CartVirtual Seminar | Jun 10, 2020
-
Add to CartVirtual Seminar | Jun 3-4, 2020
-
Add to CartVirtual Seminar | Jul 6-7, 2020
-
Add to CartSan Francisco, CA | Oct 22-23, 2020
-
Add to CartVirtual Seminar | Jul 9-10, 2020
-
Add to CartVirtual Seminar | Jun 3-4, 2020
-
Add to CartVirtual Seminar | June 3-4, 2020
-
Add to CartMiami, FL | Jul 29-31, 2020
-
Add to CartVirtual Seminar | Jun 17, 2020
-
Provider: ANSIAdd to CartPrice: $142
- Add to Cart
- Add to Cart
- Add to Cart
-
Provider: ANSIAdd to CartPrice: $120
-
Provider: ANSIAdd to CartPrice: $250
-
Provider: SEPTAdd to CartPrice: $299
- Add to Cart
-
Provider: Quality-Control-PlanAdd to CartPrice: $37
- Add to Cart
-
Provider: At-PQCAdd to CartPrice: $397
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart








